To gain a better understanding
of over-fertilization, we need to know more about water, its properties
and characteristics. Organisms utilize nutrients in the water and
their distribution is dependent of currents and layers in the water.
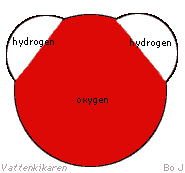
A water molecule contains one oxygen atom (O) and two hydrogen atoms
(H), and therefore has the formula H2O. |
The ends of the molecule have
different electrical charges. The hydrogen ends have equal positive
charges, while the oxygen end has a negative charge. This results
in waters ability to bind many different elements and molecules because
the different ends are able to bind to different parts of other molecules.
Ordinary salt, sodium chloride, is a substance that easily dissolves
in water because the positive sodium ions are drawn to the oxygen
end of the water molecule, while the negative chloride ions are drawn
to the hydrogen ends. Ions are atoms or
groups of atoms that either pick up or discard electrons so that they
have been charged electrically.
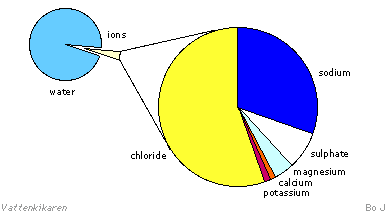
Many vitally important
soluble nutrients in seawater are in ionic form. The ion content
can vary greatly and sometimes be very low. However, the number of
ions vary very little in relation to each other. The diagram below
shows the quantities of the different ions in seawater.
|